A One-Stop Shot for Curing Diabetes
Terms to know:
Blood Glucose– The sugar found in the blood and the body’s main source of energy. Also called blood sugar.
Insulin– A hormone that helps the body use glucose for energy and storages.
Pancreas– An organ where insulin is produced and release in the blood stream in response of increased glycemia after a meal.
Beta Cell– Insulin producing cells inside the islets, also one of the few types of cells with glucokinase. Glucokinase enzyme is sensitive to plasma glucose increased.
Receptor– Molecule present on the cell surface membrane that can bind specific ligands (such as hormones) and active intracellular pathways functions.
Gene– A sequence of DNA, or nucleotides, which encodes hereditary information on the characteristics of the organism.
Genome– A person or organisms complete set of genes. (Each cell has the full genome, but due to cell differentiation each type of cell only express certain genes in the genome. For example, each cell has the genetic info to become a skin cell, hair cell, liver cell, but differentiation turns the “liver genes” off in the skin cell and the “skin genes” in the liver cell. All genes of the genome is present in all cells but only specific set of genes are expressed depending the type of cells i.e. liver, skin, kidney etc.)
CNIP Cocktail– Cocktail is composed of two genes and one molecule of interest inside lentivirus vectors used to integrated the CNIP cocktail into the pancreatic cells genome to induced formation of pancreatic insulin releasing cells.
In vivo– Tests that occur in a living animal/organism.
Background
When digesting food, the body breaks down the nutrients into smaller and smaller units. Depending on the type of molecule (ex: fat, carbohydrate, sugar), the body absorbs the nutrients at different points along the digestive track (specifically the small intestine for glucose). Once digestion has occurred, most nutrients travel via the body’s blood system to spread throughout the body to be used by all cells; the amount of sugar traveling throughout the body at a given time is what is measured when someone gets their blood sugar tested.
The cellular uptake of sugars, including glucose, is regulated by insulin and happens in specific tissues (liver, muscle and adipocyte (fat) tissues). Insulin binds a specific receptor on a cell’s surface membrane. When insulin binds to the receptor, glucose enters the cell from the blood stream—if the insulin receptor isn’t activated, the amount of sugar in the blood remains elevated. Insulin is synthesized, stored and released by a specific type of cell in the pancreas, called beta cells.
Type 1 diabetes can occur at any age, but is most commonly diagnosed from infancy to late 30’s. If a person is diagnosed with type 1 diabetes, their pancreas produces little to no insulin, and the body’s immune system destroys the insulin-producing cells in the pancreas (JDRF.org, 2017). Insulin replacement (usually self-administered insulin injections) becomes a necessary therapy to regulate glucose uptake in type 1 diabetics, and a subgroup of type 2 diabetes that need insulin injection.
A problem with insulin injection replacement is that it does not produce and release insulin minute to minute after a meal as in normal pancreatic beta cells. Consequently, hypoglycemia and hyperglycemia are still present with insulin injection. This will lead to the development of diabetic complications in many individuals.
What Are Scientists Doing
Less than 100 years ago, Frederick Banting and Charles Best discovered insulin at University of Toronto. Before then, it was a death sentence to be diagnosed with diabetes. We are still learning today from a treatment in type 1 diabetes dating form 1922. Many lives were saved by this discovery of insulin, which gave us the treatment of insulin injection. However, the injection of insulin never will be able to reproduce the natural secretion of insulin in response to an elevation in blood glucose concentration.
In the last few years many scientists have tried different therapeutic approaches for both type 1 diabetes and type 2 diabetes. Recently, at The University of Texas Health Science Center at San Antonio’s Principal Investigator Dr. Bruno Doiron, with his collaborator Dr. Ralph DeFronzo, have succeeded to generating pancreatic insulin producing cells and cure a diabetic rodent model for more than one year with any side effects and hypoglycemia.
Why the Approach Is Novel
Dr. Doiron et al. chose to call the approach “Cellular Networking, Integration and Processing (CNIP)” to emphasize the novelty in the synergy of their method. “Synergy is the key factor, in which multiple molecules work together to produce an effect that is greater than the sum of their individual effects,” says the 2016 article.
The CNIP cocktail targets three levels intracellular pathways. The first action of the cocktail is to increase the level and activity of an enzyme called glucokinase. This enzyme is present in liver cells, beta cells and in specialized gut cells. Glucokinase is important for sensing the glucose (sugar) levels in the blood, and triggering the release of insulin.
The second action of the CNIP cocktail is to activate membrane receptors associated with pancreatic beta cell formation. Pancreatic beta cells can form in response to physiological stress or pregnancy by responding to hormones in the bloodstream (prolactin, placental lactogen, etc.). These hormones and other hormones, like insulin, can directly or indirectly activate receptors (called tyrosine kinase receptors); these receptors have been shown to be influential in signaling for the body to create beta cells, although increasing the amount of tyrosine kinase receptors activities alone did not induce pancreatic beta cell formation.
Protein-tyrosine phosphatase 1B (PTP1B), has been shown to inhibit these key tyrosine kinase receptors needed for beta cell formation. Therefore, it seems fitting that part of the CNIP approach targets the PTP1B molecule with a shRNA, which is a construct that degrades the PTP1B before it can inhibit these receptors (therefore keeping the receptors available, and ready to be activated by the binding hormones).
Transcription factors are proteins that bind the DNA to regulated gene expression. The transcription factors help coordinate the effects of up regulating glucokinase activity and down regulation of PTP1B to synergize all the effects to form pancreatic insulin producing cells.
Alone, targeting each of these levels separately of cellular function does not increase insulin producing cells mass. Just separately: [1] increasing glucokinase, [2] decreasing PTP1B (thereby increasing tyrosine kinase receptors available), [3] up regulating transcriptions factors, the results do not add to the same significance as when all the three molecules of the CNIP cocktail are administered at the same time (i.e. This is the synergistic effect when all the molecules work together to induces insulin producing cells formation).

How it works
Viruses are non-living vectors that carry DNA and cell machinery in a casing, and integrate themselves
into a host organism, and use the host cell’s machinery, energy, and building blocks to replicate and infect more cells. Scientists have begun to harness the virus’s ability to infiltrate host cells and integrate themselves into the host genome, without the host developing the original virus the vector was derived from.
There are different types of viral vectors that can be utilized. Previous attempts not only differed in approach but also diabetic model. This novel therapy employs a lentivirus, which ensures long lasting integration of the new genes into the host genome (biology.kenyon.edu).
Dr. Doiron et al. have added genetic information to a lentivirus vector, which encodes genes that target all the levels of the cellular process (previously discussed) to activate cascades of intracellular signal to the cell to create insulin producing cells. The viral vector CNIP cocktail is injected specifically into the pancreas. The host cells, which are the functioning pancreatic cells (not beta cells), integrate the viral vector with the target gene therapies into their genomes. The CNIP cocktail mimic the natural intracellular pathway uses by pancreatic cell to induced insulin producing cells.
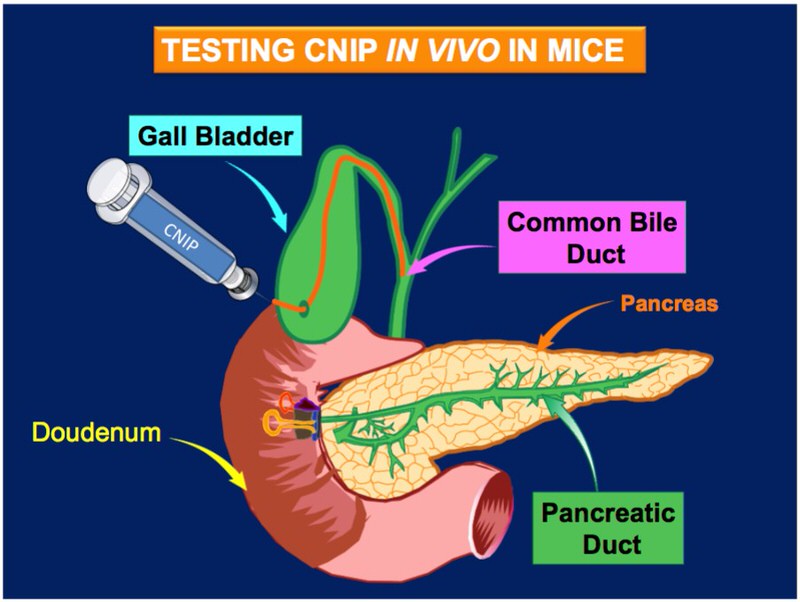
Diabetes symptoms appears when more than 80% of pancreatic beta cell are destroyed by the immune systems attack. Dr. Doiron’s induced diabetic mouse model by removing 80 percent of the pancreas that mimic the pancreatic beta cell lost in type 1 diabetes. There are other diabetic mouse models, Dr. Doiron choose to use this diabetic mouse model of partial pancreatectomy to eliminate confounding effects present in other diabetic mouse models. The CNIP cocktail was injected specifically into the pancreas. “A major advantage of the CNIP cocktail is only 20-25 percent of new insulin producing cells formation is need to cure type 1 diabetes,” says Doiron’s 2016 article.
Relevance
According to the American Diabetes Association, “In 2015, 30.3 million Americans, or 9.4 percent of the population, had diabetes. Approximately 1.25 million American children and adults have type 1 diabetes.”
The World Health Organization reports, “The global prevalence of diabetes among adults over 18 years of age has risen from 4.7 percent in 1980 to 8.5 percent in 2014. Almost half of all deaths attributable to high blood glucose occur before the age of 70 years. WHO projects that diabetes will be the seventh leading cause of death in 2030.”
While these facts make the need for a cure for diabetes obvious and urgent, Dr. Doiron emphasized the detrimental effects that diabetes causes that go unaccounted for. In addition, insulin injection is prone to complications associated with repeated episode of hypoglycemia (neurologic dysfunction) and chronic hyperglycemia (retinopathy, nephropathy, neuropathy).
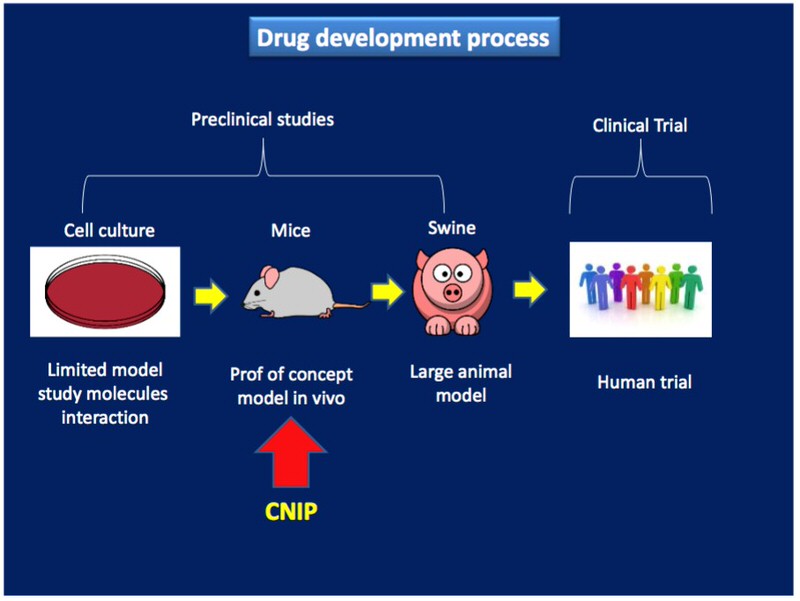
This publication contains many groundbreaking techniques not only for possible cures and treatments for diabetes, but also for a better mouse model than is sometimes used to model diabetes. This model “eliminates the confounding variables associated with the use of other mouse models… previously employed to examine beta cell formation in vivo,” says the 2016 Doiron article.
A better mouse model means that researchers can more effectively study possible treatments, without wasting animal or human clinical trials on a less than ideal therapy.
This approach is novel in the use of a lentivirus (as opposed to an adenovirus) to introduce a long lasting synergistic therapy that affects three cellular levels of regulation.
The FDA has recently approved the first lentivirus vector treatment that cures a rare type of leukemia. Dr. Doiron’s approach uses the same lentivirus as a taxi to transport therapeutic cDNA and shRNA molecules into the pancreatic cell genomes. It has shown to be successful already in a young person who has now been in remission for over five years of leukemia due to this gene transfer treatment with lentivirus vector. This helps pave the way for viral vector therapies to begin getting to the people that need new innovative and effective treatments.
“The present results provide a novel approach to the generation of beta cells using the natural physiologic cellular capacity of adult cells in the pancreas to form beta cells in vivo. The CNIP cocktail has the potential to be used as a preventive or therapeutic treatment or cure for type 1 diabetes and type 2 diabetes. The lentivirus injection method employed can be used to target pancreatic cell to induce the formation of insulin producing cells,” Doiron says in the article (2016).
The more we learn about biology on a molecular level, the more we are prone to only look at one mechanism at a time—but our body does not work like that. People with diabetes, and even those without, can benefit from Dr. Doiron and his colleagues’ findings the synergistic approach. Employing the newly approved viral vectors as possible treatment options effectively paves the way for therapies or cures for many diseases and abnormalities.

Find the full text at:
https://www.ncbi.nlm.nih.gov/pubmed/26696016
To find out more about Doiron et al., vision of curing diabetes, go to:
www.syner-iii.com (all slideshow pictures from here)
Reference material: (https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024703/)
About the Author
 MaryAnn is currently a research assistant in the Cellular and Integrative Physiology department, with hopes of attending a Biochemistry and Structural Biology graduate program in fall 2018.
MaryAnn is currently a research assistant in the Cellular and Integrative Physiology department, with hopes of attending a Biochemistry and Structural Biology graduate program in fall 2018.

