2019 HHS SBIR/STTR Grant Omnibus Solicitations Now Available!
The 2019 HHS SBIR and STTR Omnibus grant solicitations are now available!
The next deadline is September 5, 2019.
The Department of Health and Human Services (HHS) has just released the 2019 Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Omnibus Grant Solicitations! These solicitations will be used by the National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA). Note STTR is only available at NIH. You can read more in NOT-OD-19-105: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-19-105.html
The HHS SBIR and STTR Omnibus grant solicitations permit researcher-initiated topics around health, medicine and life science to be submitted for funding consideration. With any specific idea, you should speak directly with a HHS SBIR/STTR program manager at least a month BEFORE the deadline to gauge their interest.
NIH Matchmaker can be used to conduct keyword searches of a particular topic area to identify NIH Institutes or Centers (ICs) that have previously funded research in that area. Applicants can also search the 2019 Program Descriptions and Research Topics document to identify priority research areas of ICs, or email sbir@od.nih.gov for an SBIR/STTR program manager referral.
The 2019 SBIR/STTR Omnibus solicitations and accompanying resources can be found below:
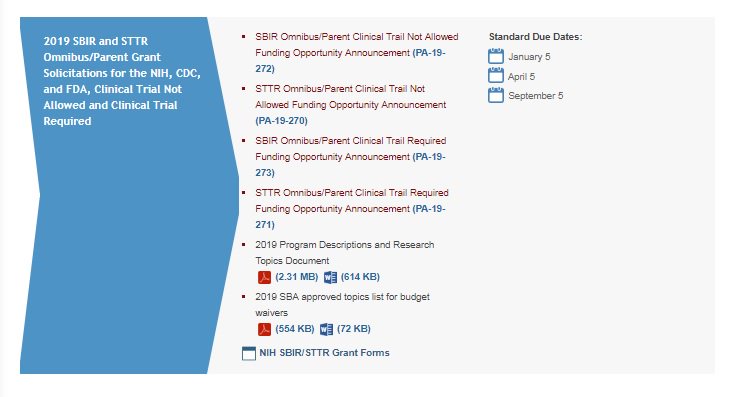
- PA-19-272 – PHS 2019-02 Omnibus Solicitation of the NIH, CDC, and FDA for Small Business Innovation Research Grant Applications (Parent SBIR [R43/R44] Clinical Trial Not Allowed
- PA-19-273 – PHS 2019-02 Omnibus Solicitation of the NIH, CDC, and FDA for Small Business Innovation Research Grant Applications (Parent SBIR [R43/R44] Clinical Trial Required
- PA-19-270 – PHS 2019-02 Omnibus Solicitation of the NIH for Small Business Technology Transfer Grant Applications (Parent STTR [R41/R42] Clinical Trial Not Allowed
- PA-19-271 – PHS 2019-02 Omnibus Solicitation of the NIH for Small Business Technology Transfer Grant Applications (Parent STTR [R41/R42] Clinical Trial Required
- 2019 Program Descriptions and Research Topics, and Appendix A (SBA approved topics for budget waivers) (https://sbir.nih.gov/sites/default/files/2019-2_SBIR-STTR-TOPICS%20FINAL.pdf)
- NIH SBA-Approved SBIR/STTR Topic Budget Waivers (https://sbir.nih.gov/sites/default/files/NIH_Topics_for_Budget_Waivers.pdf)
- SF 424 (R&R) PDF Guide Application Guide for SBIR/STTR Grant Applications (http://bit.ly/SBIRAppGuide)
- Annotated SF424 SBIR/STTR Form Set (http://bit.ly/Annotated_FormsE)
Standard Application Due Dates: September 5, 2019, January 6, 2020, and April 6, 2020 (since January and April 5, 2020 fall on a Sunday)
An informational webinar will occur on Wednesday, May 29, 2019 at 1:00 PM to 3:00 PM EDT.Please register for the HHS SBIR/STTR PHS 2019-2 Grant Omnibus Webinar on May 29, 2019 at 1:00 PM EDT:
https://attendee.gotowebinar.com/register/483596398390515457
After registering, you will receive a confirmation email containing information about joining the webinar.
You can find the HHS SBIR/STTR Omnibus Solicitations, and all of our other SBIR/STTR solicitations on the Funding page of our website: https://sbir.nih.gov/funding. Be sure to also check the targeted funding announcements page, https://sbir.nih.gov/funding/individual-announcements. NIH Institutes and Centers will issue targeted SBIR/STTR grant solicitations around specific, high-priority research areas.
For specific questions about the HHS SBIR/STTR programs, please contact sbir@od.nih.gov.
Follow us on Twitter: @NIHsbir (https://twitter.com/NIHsbir)
Connect with us: http://sbir.nih.gov/engage#connect