Inflammation, The Gut Microbiome, And Osteoarthritis: What Is The Link?

We hear daily that what we eat affects our health and the importance of eating “healthy.” Eating healthy is different from person to person as diets are complex but what we eat may actually help in the management of a disease. Various diets have been trending recently such as the keto and the anti-inflammatory diet which focus on how inflammation plays a large role in obesity and many diseases including osteoarthritis. Osteoarthritis (OA) is a degenerative joint disease, where the cartilage tissue lining the ends of articulating joints deteriorates, which affects more than 30 million adults in the U.S. according to the CDC. People affected with osteoarthritis suffer from stiffness, and chronic pain in their joints primarily the knee and hip, and eventually with progression of the disease a restriction in movement. Unfortunately, the management of osteoarthritis up till now has been the use of pain killers as the front-line defense with some doctors also prescribing lifestyle changes and physical therapy, until a total knee replacement surgery is the only option left. While these surgeries restore functionality and movement, they are invasive and have a significant recovery time. At the same time due to active lifestyles younger people are being diagnosed with OA and currently total knee replacement surgeries are not recommended for younger patients, due to the risk of needing a secondary surgery.
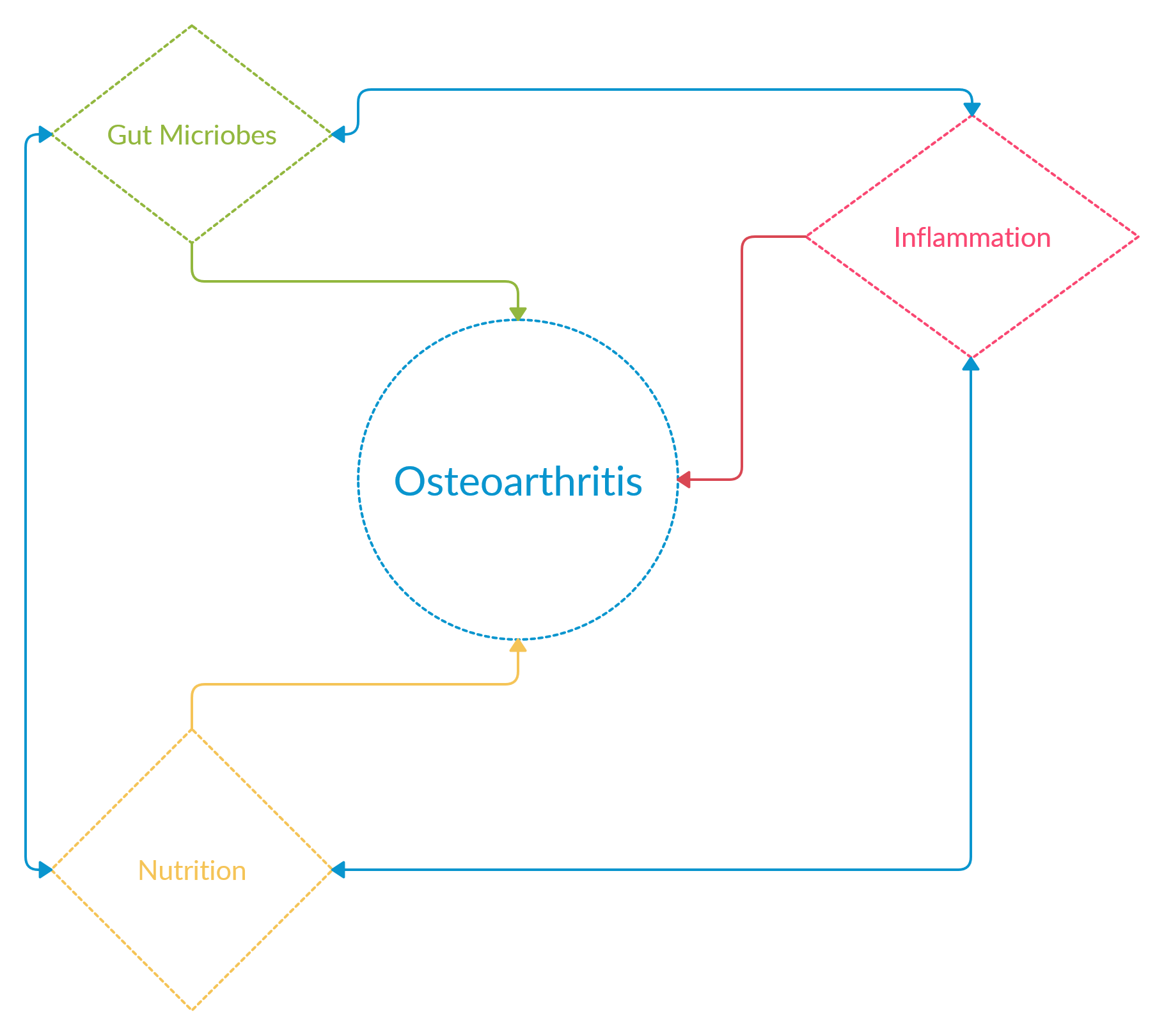
Thus, preserving our joints and maintaining the cartilage tissue for as long as possible is highly recommended and seems to be the best option. As such, there is growing interest in seeking sustainable, health-conscious approaches to OA management in the form of alternative treatments that may halt OA progression, maintain cartilage tissue, and reduce inflammation, could nutritional supplements be part of the solution?
Inflammation
Osteoarthritis as a whole joint disease is influenced largely by the inflammatory response due to cartilage injury and an inflamed synovium, the lining encapsulating the joint. Studies in the last few years have started to highlight the role that inflammatory cytokines such as interleukin-1-beta and tumor necrosis factor alpha play in triggering a cycle of pain and further progressing tissue degeneration. Upon injury, inflammatory cytokines are released stimulating matrix metalloproteinases (MMPs) production, which degrade the extracellular matrix (ECM) components that make up a tissue. The inflammatory response is usually stimulated by an injury to trigger a cascade of responses that releases inflammatory cytokines, reactive oxide species and MMPs to remove debris or fragments of tissue from the site of the injury and then initiate a rebuilding response to remodel the injured tissue to heal. This works well for vascularized tissue, that has blood vessels, which aids in remodeling the tissue. Yet cartilage tissue is avascular, meaning it does not have blood vessels, and when there is a tear in cartilage due to injury it similarly triggers an inflammatory response and MMP activity. Major ECM components such as collagen type two or aggrecan released due to degradation, often acts a stimulant in a feedback loop for further MMP activity and tissue degradation1. Cells are also released to the site of the injury to remodel the tissue, yet this process is slow in comparison to the ongoing degradation. As such in chronic inflammation the trigger or stimulus for the catabolic process or removal of tissue fragments is unceasing not allowing the response to move to the tissue remodeling phase or anabolic process of building new tissue. Chronic inflammation, the body’s natural response to injury, thus plays a role in the cycle of further degrading the tissue and progressing OA. Suppressing the activity of interleukin-1-beta and tumor necrosis factor alpha has been shown to reduce MMP production, slow cartilage destruction and reduce inflammation1.
Gut microbiome
How can the gut microbiome play an essential role in halting degenerative diseases? The microbiome consists of the entire ecosystems of microbes such as bacteria that live within or on humans. Interestingly we have more bacterial cells than we do human cells in our body. A healthy microbiome may be different from one person to the next however drastic changes or imbalances in the microbiome have been linked to inflammatory diseases such as Parkinson’s disease, rheumatoid arthritis, type 2 diabetes, and osteoporosis2. The common factor in all these diseases is the presence of chronic inflammation. Interestingly the gut microbiome acts a regulator of inflammation by producing inflammatory cytokines and bacterial metabolites such as lipopolysaccharides (LPS). Increased LPS levels have been tied to OA severity, as well as linking the results to increased levels of the microbe Firmicutes2. There have been a couple of studies on murine and rat models that have linked high fat or sugar diets with higher LPS levels leading to increased cartilage damage or higher OA severity and increased levels of the microbe Firmicutes as well as 2 species of bacteria that are associated with obesity2. Firmicutes and Bacteroidetes are the main species in the microbiome, with the ratio of higher Firmicutes to Bacteroidetes associated with proinflammatory states, or an imbalance of the gut microbiome often referred to as gut dysbiosis. Gut dysbiosis has also been linked to obesity, which is a factor in OA as well. In a human clinical trial with patients with knee OA they found an association between higher scores on the OA scale and proinflammatory microbes or bacteria species in the gut2.
Dietary supplements and nutrition
Understanding the effects of inflammation and the imbalance in the gut microbiome on the progression of OA, we can see the complex interplay between them. Dietary patterns and obesity have been linked with severity of OA and have shown promise in reducing the risk of OA with changes to eating habits3. Targeting the chronic inflammation associated with OA is one method that is being pursued through the use of supplements such as nutraceuticals which are natural or synthetic compounds, vitamins, herbs or tea extracts that have health benefits in managing a disease. Numerous invitro studies have found the use of nutraceuticals, such as vitamin E, vitamin C, and green tea extracts, to decrease inflammatory markers in OA models4-7, yet the mechanistic action of how nutraceuticals would achieve this in a clinical aspect has not been understood. A recent review linked the gut microbiome and its inflammatory regulator role to the action of nutraceuticals on OA severity and inflammation2. In the review they discuss studies that have shown links between gut dysbiosis and OA progression. For example, rats fed on a high fat diet were found to have gut dysbiosis and lacked a bacteria species that has anti-inflammatory properties, when the rats were supplemented with an indigestible fiber the bacterial species was restored and cartilage degradation was halted in comparison to rats only on the high fat diet2. At the same time proinflammatory bacterial species were completely reduced when supplemented with indigestible fiber. Several dietary supplements containing nutraceuticals such as collagen, and chondroitin sulfate, are marketed towards OA relief. Studies on murine and rat models have shown that supplementation with collagen mitigated OA progression and joint degeneration in rat and murine models2. While interesting, the gut microbiome connection or the mode of action of the nutraceuticals was not understood in those studies. A study to explore that effect, created a post-traumatic OA mouse model that were either fed with glucosamine, or undenatured type 2 collagen, or the control vehicle. The main findings showed mice fed with either nutraceutical had less OA severity scores compared to the control, and slower cartilage degeneration as shown by histological samples showing cartilage coverage on the joint. Upon analysis of the gut microbiome they found a higher ratio of Bacteroidetes to Firmicutes in nutraceutical supplementations compared to the control which shows a shift towards an anti-inflammatory state2. This study along with others highlights the possibility of biological action of nutraceuticals impacting joint health through modulation of the gut microbiome.
Briefly, we understand that all diseases are multi-factorial and osteoarthritis similarly has a complex interplay between inflammation and continued tissue degradation. Targeting the gut microbiome thorough the use of nutraceutical supplementation seems to be a promising avenue for understanding how supplements affect joint health providing a mechanistic understanding of biological action seen in invitro studies and nutraceuticals may aid in an overall reduction in inflammation, halting OA progression, and maintaining cartilage tissue for a longer period.
About The Author
 Alia Mallah is a doctoral student in the joint Biomedical Engineering Program at the Graduate School of Biomedical Sciences. Her current research focuses on understanding the inflammation associated with osteoarthritis and using tissue engineering techniques for alternative treatment options. She is interested in tissue engineering techniques, cell culture, nutraceuticals, scaffolds, microscopy and colorimetric assays.
Alia Mallah is a doctoral student in the joint Biomedical Engineering Program at the Graduate School of Biomedical Sciences. Her current research focuses on understanding the inflammation associated with osteoarthritis and using tissue engineering techniques for alternative treatment options. She is interested in tissue engineering techniques, cell culture, nutraceuticals, scaffolds, microscopy and colorimetric assays.
References
- Woodell-May JE, Sommerfeld SD. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J Orthop Res.2020;38:253-257.
- Favazzo LJ, et al. The gut microbiome-joint connection: implications in osteoarthritis. Curr Opin Rheumatol.2020;32:92-101.
- Xu C, Marchand NE, Driban JB, McAlindon T, Eaton CB, Lu B. Dietary Patterns and Progression of Knee Osteoarthritis: Data from the Osteoarthritis Initiative. Am J Clin Nutr.2020;111:667-676.
- Castrogiovanni P, Trovato FM, Loreto C, Nsir H, Szychlinska MA, Musumeci G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int J Mol Sci.2016;17.
- Chin KY, Ima-Nirwana S. The Role of Vitamin E in Preventing and Treating Osteoarthritis – A Review of the Current Evidence. Front Pharmacol.2018;9:946.
- Henrotin Y, Lambert C, Couchourel D, Ripoll C, Chiotelli E. Nutraceuticals: do they represent a new era in the management of osteoarthritis? – a narrative review from the lessons taken with five products. Osteoarthritis Cartilage.2011;19:1-21.
- Chang Z, Huo L, Li P, Wu Y, Zhang P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep.2015;12:7086-7092.
