Why Age May Just Be A Number: The Epigenetics of Aging

Figure 1: A photo captured during the Dutch famine
In the winter of 1944-1945 in the Netherlands a great famine occurred due to the German occupation. This led to many people having to consume as little as 1000 calories a day for many, many months. The recommended intake for a healthy adult person is, on average, 2300 calories a day but during this period people were eating as little as 1000 calories a day.
This famine led to epigenetic changes occurring in the women who were pregnant at the time, causing the unborn foetus to adapt to the lower available amounts of sustenance. The babies born during this period were followed throughout their lives and studies showed that once they had reached their sixties, they showed a much higher level of chronic conditions such as diabetes and cardiovascular diseases, than people born in less troubled times. This study shows one of the ways that changes in our environment can impact the way our genes function and can have long term effects on our health and lifespan.
Your genetic code is the same throughout your body but in every organ your cells need to perform a different function. Epigenetics is the study of how these biological molecules which interact with your genes, can affect the way that the genetic code is expressed. In some instances, this can lead to certain genes in your cells being turned off, where they serve no purpose, or on when they are needed. For example, certain proteins which promote bone growth are not produced in muscle cells, despite the cell having the same genetic material as an osteoblast cell, which in other places in the body is responsible for producing bone. A good analogy is to think of your genome as the hardware of the computer and the epigenome (the record of chemical changes to the DNA and histone proteins) as the software. This is because the epigenome is what helps to tell our cells what kind of cells, they should be so they can complete their task. Part of the reason why this exists is that your DNA doesn’t know what kind of environment you’re born in so your epigenetics exists as a tool to help adapt you to the situation you’re in such as in the Netherlands famine where the foetuses had to adapt to lower levels of calories.
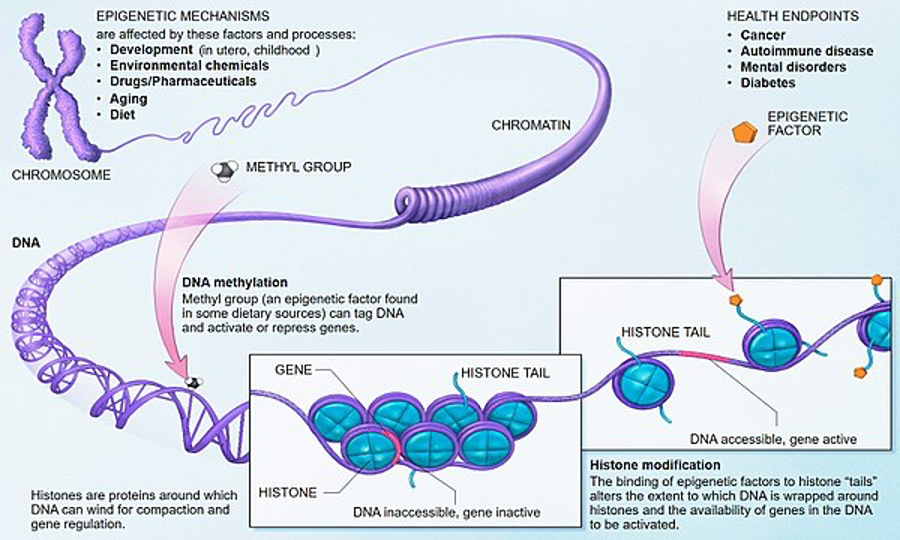
Figure 2: A more detailed look at how epigenetic changes occur on the microscopic scale
Aging causes a multitude of processes in your body to begin their slow decline in performance. Your heart slowly reduces its capacity to pump the same volume, and your bones’ mineral content to decrease, so that they become less dense and more fragile. Also, as you age your risk of developing various metabolic diseases such as cancer, neurodegeneration and cardiovascular disease increase dramatically. In the face of this somewhat bleak outlook, what we want to focus on is not just improving our lifespan but our ‘health span.’ Your health span is the part of your life where you are generally considered to be in good health. On average in the U.S. the health span is around 63 years, meaning approximately 16 years of your life is, on average, in bad health – a staggering, or disappointing 20 percent of your life. If there was a way to improve both our lifespan and health span it would mean we could live more of our lives happy, healthy, productive, benefiting not only ourselves but reducing the perceived burden on society. By researching the links between epigenetics and aging it is hoped that some of the negative changes that take place on your epigenome could hopefully be either slowed down, decreasing some of the effects of aging or possibly even prevented. We will be getting science to help change a downward progression that a change in lifestyle can only partially effect.
Research is mainly in its early stages and we need to develop a better picture of how our epigenome and the aging process fit together. We also need to be looking into finding particular enzymes which would be able to work as targeted therapeutic treatments to help to improve our health and lifespan but to find these it would require a better idea of the biochemical mechanisms that take place to cause epigenetic changes to occur.
Current researchers focus on improving single disease state and multiple disorders of aging, through epigenetic drugs/epigenetic diets. Although the emerging concept of developing epigenetic drugs or epigenetic diet is an intriguing idea, one of the major challenges in this field is to determine specificity of epigenetic therapies because mechanisms regulating epigenetic information are interconnected meaning that one change could have multiple possible negative ripples.
Whilst research in epigenetics is usually geared more towards understanding and treatments, an experiment in the U.S. by prominent age researcher Steven Hovarth and a team of fellow scientists tracked a process called DNA methylation in patients cells, which is an epigenetic modification which can switch off genes. The group’s findings showed how the epigenetic clock can predict when somebody will die more accurately than their real age even if risk factors are considered such as disease history and BMI are considered. This shows how if our understanding of epigenetics were to improve, we may be able to exploit and interfere with biological mechanism in ways we never thought possible.
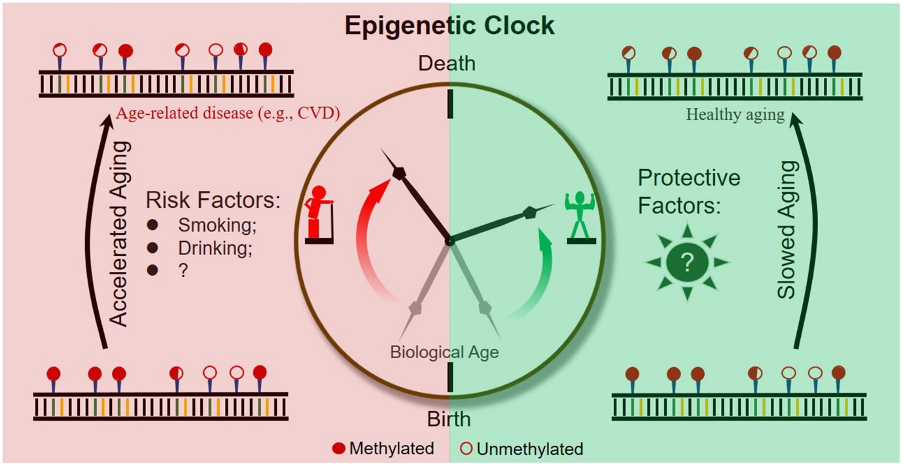
Figure 3: A schematic diagram of an epigenetic clock across a human lifetime.
Hopefully, years down the road we will have been able to develop targeted therapies to counter many of the side effects of aging such as the increased risk of chronic diseases and even the wrinkles on your face. It could even mean that age becomes a thing of the past once we hack the human biology, administering the right drugs and treatments, to the point where life could seem almost infinite. The old adage of ‘a life well-lived’ will become less of conciliatory utterance and a genuine statement on a life full of good health, productivity and value.
References:
- Ahmed, Farooq. “Epigenetics: Tales of adversity.” Nature7327 (2010): S20-S20.
- Chris Wood. Forget the Genome: The Epigenome Is Where It’s At. Casey Research (Feb 2014).
- Effects of aging. Orthoinfo from American academy of orthopaedic surgeon (2009)
- Horvath, Steve, and Kenneth Raj. “DNA methylation-based biomarkers and the epigenetic clock theory of ageing.” Nature Reviews Genetics 6 (2018): 371.
- National Institutes of Health. “A scientific illustration of how epigenetic mechanisms can affect health.” (2004).
- Roseboom, Tessa J., et al. “Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview.” Twin Research and Human Genetics5 (2001): 293-298.
- The O cells. University of Washington web server. (2001)
- What is epigenetics? National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2020 Aug 17.
- Xiao, Fu-Hui, Hao-Tian Wang, and Qing-Peng Kong. “Dynamic DNA methylation during aging: a “prophet” of age-related outcomes.” Frontiers in genetics 10 (2019): 107
About The Author
Alex Brown is a 15-year-old student at Bristol Grammar School. He is interested in science and biomedicine. He was mentored by Manpreet Semwal, a Ph.D. student in our Molecular Immunology and Microbiology discipline of the Integrated Biomedical Sciences program at UT Health San Antonio to write this article.
